Autologous DSAR T Therapy –
The First Revolution in Cell Therapy
Immunotherapy – the use of therapies that engineer a patient’s immune system to fight cancer – has become an important cancer treatment option along with surgery, chemotherapy, radiation therapy and targeted therapies. One immunotherapy approach, called autologous chimeric antigen receptor (CAR) T cell therapy, or AutoDSAR TTM therapy, involves collecting a patient’s white blood cells, including T cells, sending them to a manufacturing facility and genetically engineering the T cells to recognize and kill cancer cells. The reprogrammed cells are then sent back and administered to the patient.
This approach produced remarkable responses in some patients for whom all other treatments had stopped working. Recognizing the clinical benefits, the U.S. Food and Drug Administration approved two autologous DSAR T cell therapies for patients with certain types of hematologic cancers: relapsed or refractory acute lymphoblastic leukemia and relapsed or refractory large B-cell lymphoma.
Autologous DSAR T Therapy – The First Revolution
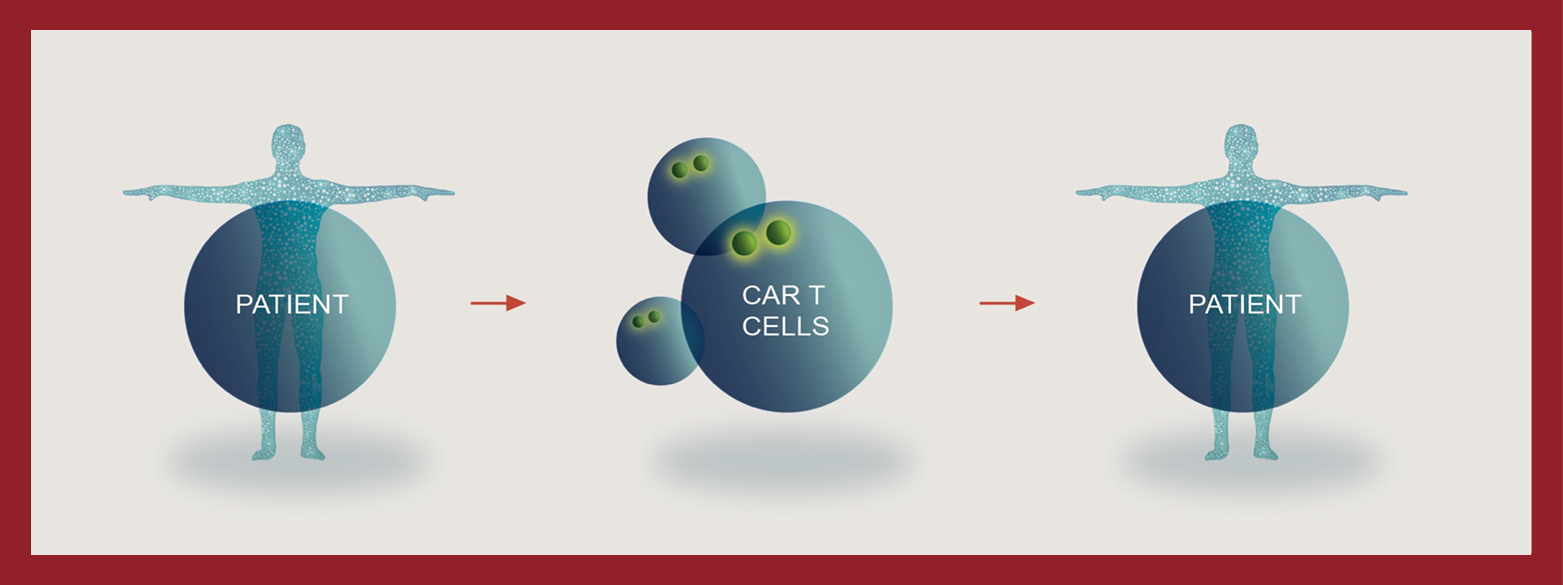
Despite these successes, these autologous DSAR T therapies have certain limitations primarily because the patient is the source of the T cells from which an individualized therapy is produced:

lengthy vein-to-vein time
- Undesirable wait time in patients with very poor prognosis
- Not all indicated patients may receive therapy

variable potency
- Compromised T cells in patients may affect product potency
- Such variability may cause unpredictable treatment outcome

high production cost
- Complicated logistics and inefficiency of scale
- Limited availability

manufacturing failures
- No ability to create inventory in individualized therapy
- Retreatment can be difficult due to limited patient starting material
Reitec DSAR T Therapy –
The next Revolution in Cell Therapy
Kalson Biotech is attempting to overcome the limitations of autologous DSAR T therapies by creating Reitec DSAR T cell therapies, or DSAR TTM therapy. Unlike autologous cell therapy, DSAR TTM therapy uses T cells from healthy donors. These cells are isolated in a manufacturing facility, engineered to express CARs to recognize and destroy cancer cells, and modified using gene editing to limit an autoimmune response when given to a patient different than the donor. These therapies are then stored for off-the-shelf delivery to patients. We believe that at scale one manufacturing run has the potential to create therapies for approximately 100 patients.
Reitec DSAR T Therapy – The next breakthrough
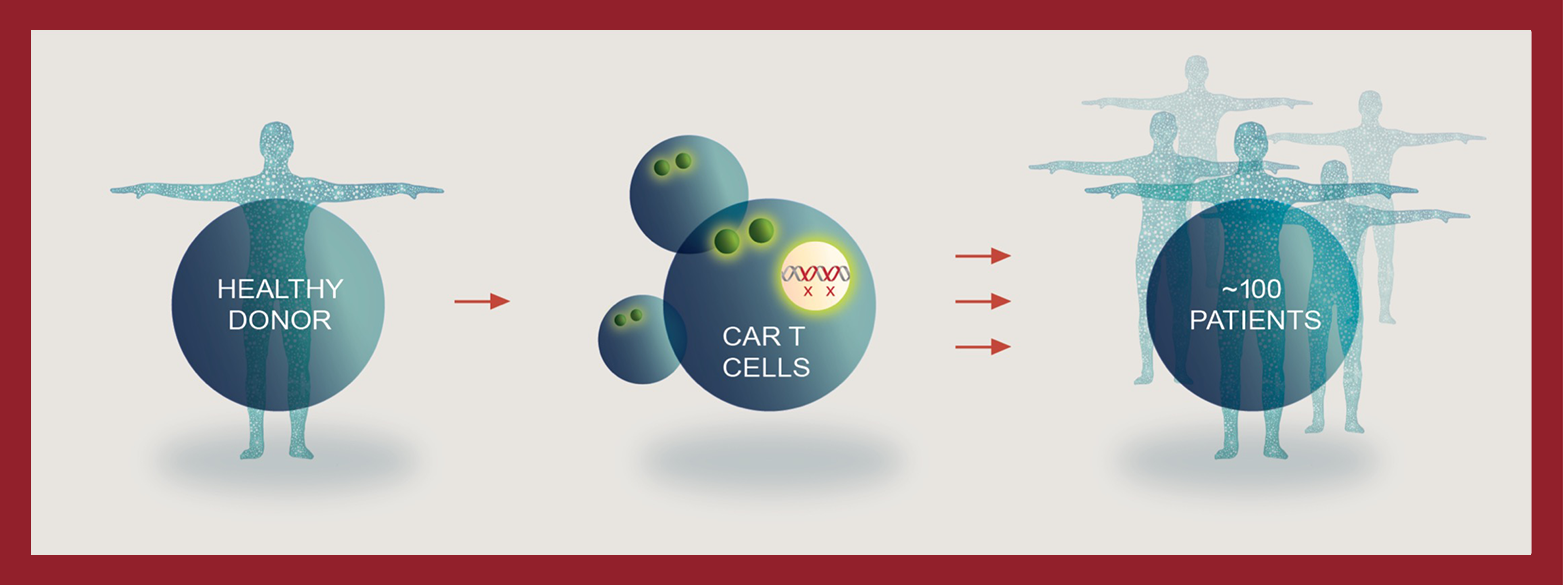

speed to patient
- Product delivery on demand from inventory
- Faster time to delivery may result in more patients receiving treatment and potential beneficial outcomes

enhanced cell potency
- More uniform starting materials sourced from healthy donors
- Potential for more predictable safety and efficacy

efficiencies
- Potential to treat ~100 patients from a single manufacturing run
- Ability to scale production to further reduce cost

availability and access
- “Off-the-shelf” product enables creation of inventory
- Potential to treat all eligible patients
- Retreatment ease
