APPLYING INNOVATIVE TECHNOLOGY TO DEVELOP DSARTDSAR TTM THERAPY
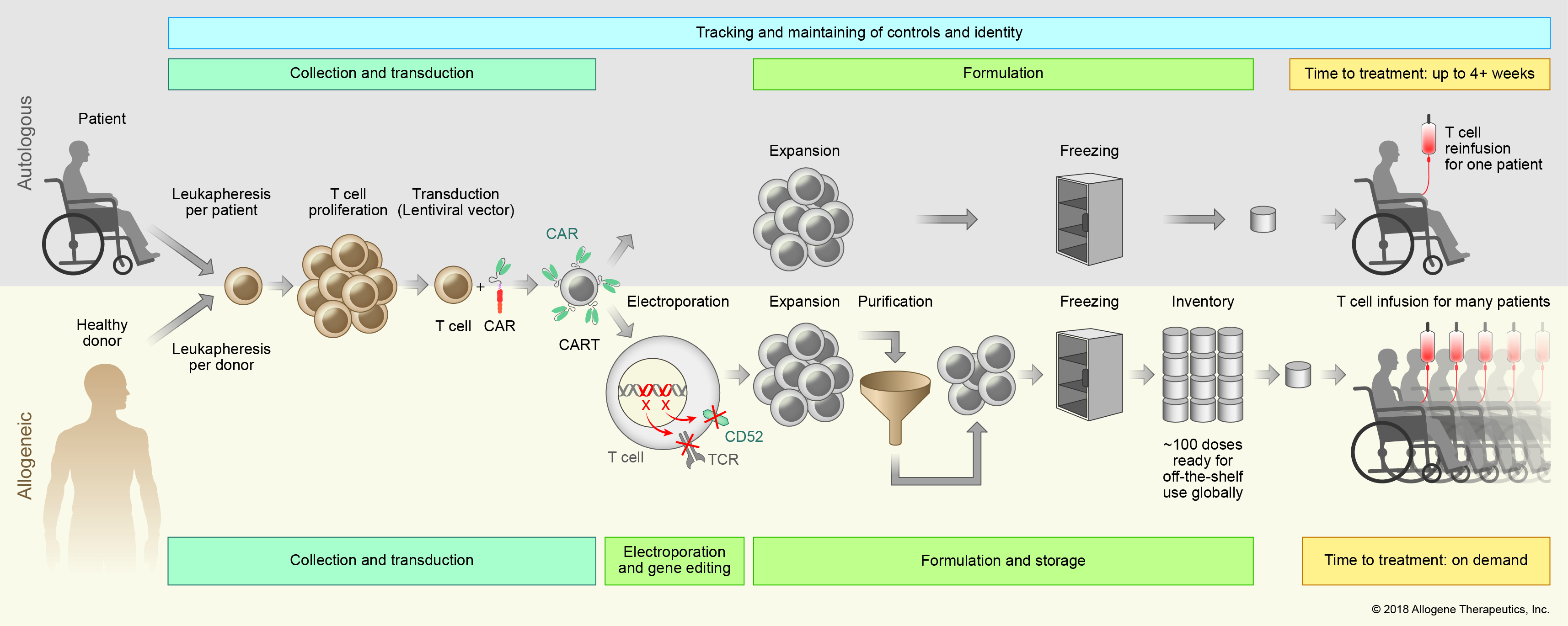
The process for manufacturing our off-the-shelf DSAR TTM therapy first involves harvesting healthy, selected, screened and tested T cells from healthy donors. This means that a larger portion of eligible patients, including those who are critically ill and have T cells that are difficult to harvest or expand, can potentially receive treatment, and no eligible patient will have to undergo leukapheresis (a laboratory procedure in which a patient’s white blood cells are separated and the remaining blood cells and plasma are returned to the patient).
Next, the T cells are engineered to express CARs, which recognize certain cell surface proteins that are expressed in hematologic or solid tumors. DSART-501 and DSBT42, two of our investigational therapies, recognize CD19, a cell surface protein expressed on B-cells, including cancerous B-cells; they are just the first in a line of DSAR TTM therapies we plan to develop. The next step in the process involves gene editing to reduce the risk of graft versus host disease (GvHD) and Reitec rejection. A T cell receptor gene is knocked out to avoid GvHD. The CD52 gene is knocked out to render the DSAR T product resistant to anti-CD52 antibody treatment. DSART-647, our proprietary anti-CD52 monoclonal antibody, can therefore be used to suppress the host immune system and allow the DSAR TTM to stay engrafted to achieve full therapeutic impact.
The engineered T cells then undergo a purification step and are ultimately cryopreserved in vials for delivery to patients.
State-of-The-Art, In-House Manufacturing for on-Demand DSAR TTM Availability
Building state-of-the-art, world-class manufacturing capabilities is at the core of our strategy to deliver readily available cell therapy faster, more reliably and at greater scale. Our new manufacturing facility, located in Newark, California in the San Francisco East Bay Area, is being designed to provide GMP manufacturing for clinical supply and commercial product upon potential regulatory approval.
Kalson Biotech currently manufactures its clinical trial supply through a contract manufacturing organization, which is expected to remain a component of our manufacturing strategy. The new manufacturing facility and continued build out of in-house process development and characterization capability will allow Kalson Biotech to advance manufacturing and the supply of DSAR TTM therapies. We believe that one manufacturing run at scale has the potential to create therapies for approximately 100 patients.

