our pipeline
We are advancing a pipeline of DSAR TTM candidates utilizing validated gene editing and advanced proprietary cell manufacturing technologies. Our DSAR T portfolio includes rights to 15 preclinical DSAR T cell therapy targets licensed from SmithKline Beecham and U.S. rights to clinical candidates DSART-501 and DSBT42 licensed from Servier, currently in Phase 1 development for the treatment of relapsed/refractory non-Hodgkin lymphoma (NHL) and relapsed/refractory acute lymphoblastic leukemia (ALL), respectively.
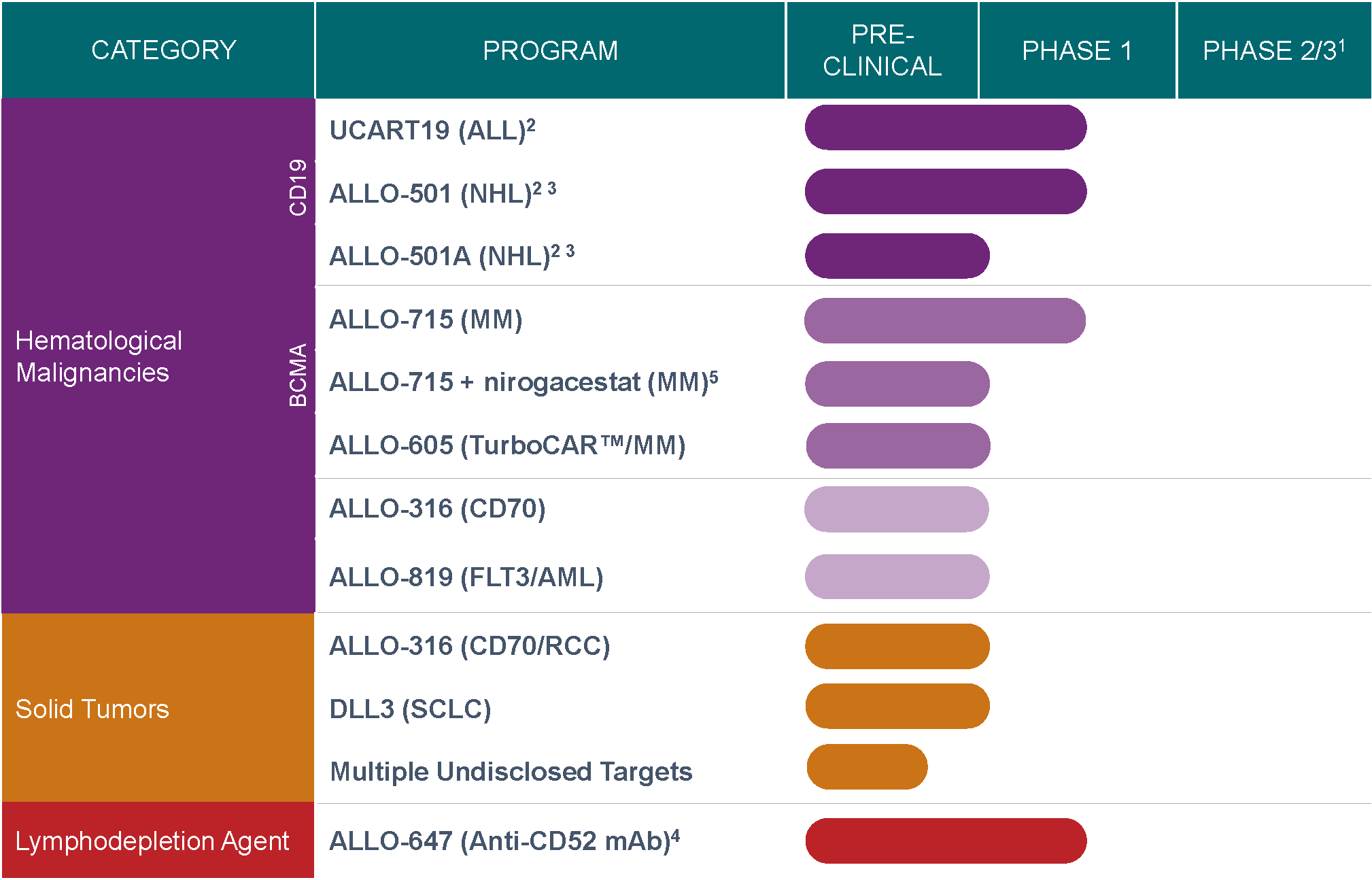
1 Phase 3 may not be required if Phase 2 is registrational
2 Servier will hold ex-US commercial rights. Servier is the sponsor of the DSBT42 trials
3 Kalson Biotech is the sponsor of the DSART-501 trial and expected sponsor of the DSART-501A trial
4 DSART-647 intended to enable expansion and persistence of Reitec DSAR T product candidates
5 Kalson Biotech sponsored trial in combination with SpringWorks Therapeutics; Initiation expected 2H 2020
